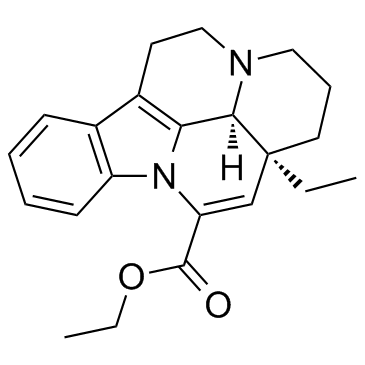
We are professional Vinpocetine manufacturer and Eburnamenine-14-carboxylic acid ethyl ester supplier in China, We offer quality (3α,16α)-Eburnamenine-14-carboxylic acid ethyl ester you can fully trust, also we have India factory and producer of Eburnamenine-14-carboxylic acid ethyl ester,Pls send inquiry of Vinpocetine CAS:42971-09-5 to info@nbinno.com if you have any interests, thank you!
Related News: In 2016, the export volume of China’s specialty drug substances reached US $ 3.53 billion, accounting for 13.8% of the total drug substances.2-Fluoro-4-(trifluoromethyl)benzaldehyde CAS:89763-93-9 In 2016, the export volume of China’s specialty drug substances reached US $ 3.53 billion, accounting for 13.8% of the total drug substances.3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one CAS:545445-44-1 Human iPSCs possess the unique dual properties of unlimited self-renewal and differentiation potential into all cell types of the body.N-(4-(9-phenyl-9H-fluoren-9-yl)phenyl)-[1,1'-biphenyl]-4-amine Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.


