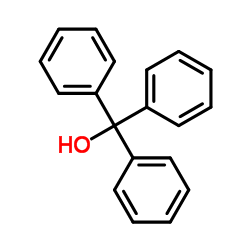
We are professional Triphenylmethanol manufacturer and 1,1,1-Triphenylmethanol supplier in China, We offer quality Hydroxytriphenylmethane you can fully trust, also we have India factory and producer of Triphenylmethanol,Pls send inquiry of 1,1,1-Triphenylmethanol CAS:76-84-6 to info@nbinno.com if you have any interests, thank you!
Related News: The Company’s first-of-kind approach involves engineering human iPSCs in a one-time genetic modification event and selecting a single engineered iPSC for maintenance as a clonal master iPSC line.2-aminonicotinic acid CAS:5345-47-1 Taking Teva as an example, it has 2 large R & D centers and 3 professional R & D centers in 5 countries, which effectively supports the development of Teva’s generic pharmaceutical sector.METHYL 3,3-DIMETHOXYPROPIONATE CAS:7424-91-1 INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carbaldehyde Inceptua Medicines Access is a business unit of the Inceptua Group. It offers full access solutions for the design, implementation and delivery of Pre-approval and Medicines Access Programs on behalf of biopharmaceutical companies.In addition, a high-standard GMP workshop is under construction and is expected to be put into production within the year. As the capacity bottleneck is broken, the pharmaceutical intermediate business is expected to grow rapidly in the future, providing the company with new growth momentum.


