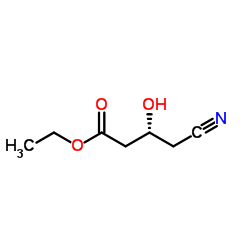
We are professional Ethyl (R)-(-)-4-cyano-3-hydroxybutyate manufacturer and Ethyl (R)-4-cyano-3-hydroxybutanoate supplier in China, We offer quality Ethyl (R)-(-)-4-cyano-3-hydroxybutyrate you can fully trust, also we have India factory and producer of (R)-(-)-4-Cyano-3-hydroxybutyric Acid Ethyl Ester,Pls send inquiry of Ethyl (R)-(−)-4-cyano-3-hydroxybutyrate CAS:141942-85-0 to info@nbinno.com if you have any interests, thank you!
Related News: The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances.2-methylbut-3-enoic acid There is fierce competition for bulk APIs and overcapacity. However, some high-profit, small-scale drugs still have a good market space.2-bromometil-3-nitrobenzoato de metilo CAS:98475-07-1 In addition, a high-standard GMP workshop is under construction and is expected to be put into production within the year. As the capacity bottleneck is broken, the pharmaceutical intermediate business is expected to grow rapidly in the future, providing the company with new growth momentum.6298-19-7 Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.A GSK spokesman said it was not yet clear by when ViiV would be able to address the FDA’s concerns.


