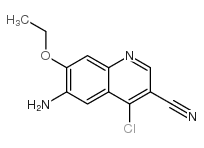
We are professional 6-amino-4-chloro-7-ethoxyquinoline-3-carbonitrile manufacturer and 6-Amino-4-chloro-7-ethoxy-3-quinolinecarbonitrile supplier in China, We offer quality 6-Amino-4-chloro-7-ethoxy-3-quinolinecarbonitrile you can fully trust, also we have India factory and producer of 6-Amino-4-chloro-7-ethoxy-3-quinolinecarbonitrile,Pls send inquiry of 6-amino-4-chloro-7-ethoxyquinoline-3-carbonitrile CAS:848133-87-9 to info@nbinno.com if you have any interests, thank you!
Related News: The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.5463-92-3 There is fierce competition for bulk APIs and overcapacity. However, some high-profit, small-scale drugs still have a good market space.2-Bromo-3-methoxypyridine CAS:24100-18-3 The Company’s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).4,4'-Diisocyanato-3,3'-dimethyl-1,1'-biphenyl CAS:91-97-4 The API is not made by only one reaction from the raw materials but rather it becomes an API via several chemical compounds.“CAR T-cell therapy continues to deliver remarkable outcomes for patients with hematologic malignancies, and next-generation approaches are needed to enable broad and timely patient access and reduce the cost and complexity of therapy,” said Scott Wolchko, President and Chief Executive Officer of Fate Therapeutics.


