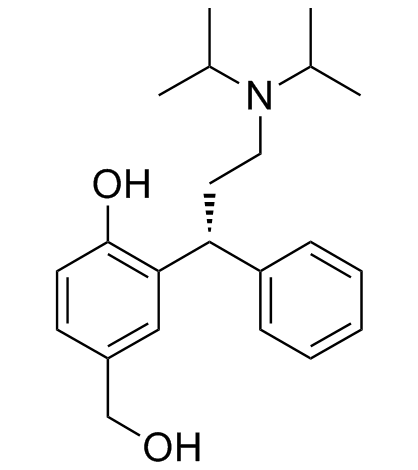
We are professional 5-hydroxymethyl Tolterodine manufacturer and 3-[(1R)-3-[Bis(1-Methylethyl)Amino]-1-Phenylpropyl]-4-Hydroxy-Benzenemethanol supplier in China, We offer quality (R)-2-(3-(Diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenol you can fully trust, also we have India factory and producer of R-5-Hydroxymethyl Tolterodine,Pls send inquiry of (R)-5-Hydroxymethyl tolterodine CAS:207679-81-0 to info@nbinno.com if you have any interests, thank you!
Related News: With the continuous accumulation of the API industry and the increasing maturity of the market, competition in China’s API industry will become increasingly fierce.3-Bromo-2-methoxypyridine The second is known as the excipient, which is the inactive substance that serves as the vehicle for the API itself.L-Serine Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.2-Methyl-5-nitropyridine CAS:21203-68-9 This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.The API can be directly formulated, and the intermediate can only be used to synthesize the next product. Only through the intermediate can the API be manufactured.


